
It can’t be b ) since that molecule lacks OH. The only option that makes sense is e) (cyclopentanol) since it has both an OH group and an IHD of 1. That broad peak at 3300 tells us that we have an alcohol (OH group).Looking at the spectrum we see a broad peak at 3300 cm -1 and no dominant peak around 1700 cm -1 (That peak halfway down around 1700 cm -1? It’s too weak to be a C=O.This immediately rules out d) whose IHD is zero and thus has a molecular formula of C 5H 12O. This corresponds to an index of hydrogen deficiency (IHD) of 1, so either a double bond or ring is present in the molecule. You’re given the molecular formula, which is C 5H 10O.Problem #4: Unknown molecule with formula C 4H 8O 2 (Also, smells like vomit) Problem #3: Unknown molecule with molecular formula C 6H 14O. Problem #2: Unknown molecule with molecular formula C 6H 12O. Which of these five molecules is it most likely to be? Problem #1: Unknown molecule with molecular formula C 5H 10O. Don’t peek until you’ve given each problem a thorough attempt). (answers to each problem, along with analysis, are at the bottom of the post.
#Infrared spectroscopy chart how to#
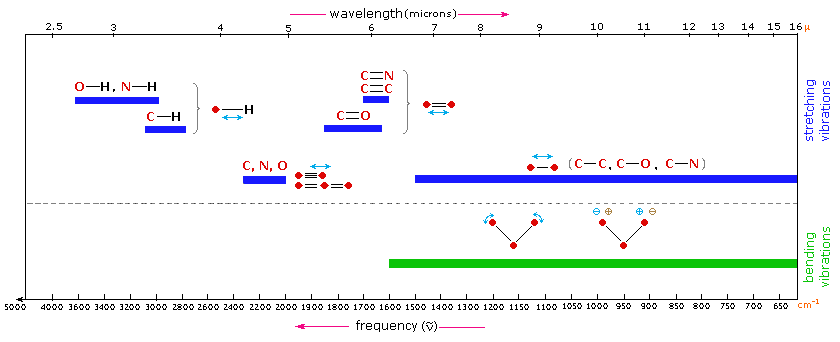
In this post we’re going to go through four (simple) practice problems where you’ll be provided with an IR spectrum and the molecular formula, and are then charged with the task of figuring out which molecule best fits the spectrum.Įverything you need to know about IR in order to solve the problems below was presented in the previous post on how to do quick analyses of IR spectra, so go back and read that if you haven’t done so already. a cyclic ester) by its characteristic absorbance at 1775 cm -1.Īdditionally, if you’ve narrowed down a structure to several possibilities, it can be very helpful in ruling various possibilities out. However, we’ve seen that IR spectroscopy can a great technique for identifying certain functional groups in an unknown molecule – especially functional groups containing OH or C=O.įor instance, in an earlier post on the structure determination of deer tarsal gland pheromone, we saw how the authors of the study used IR spectroscopy to identify the presence of a lactone functional group (i.e. By itself, Infrared (IR) spectroscopy isn’t a great technique for solving the structure of an unknown molecule.


 0 kommentar(er)
0 kommentar(er)
